SciTech-Physics-Particle-Atom:{Electron + Kernel{Proton+Neutron}} + Bands(Energy) + Movement{自由电子+空穴} + 导电 + Energy Transition: 电致发光
SciTech-Physics-Particle
Atom 组成+结构:
-
Nucleus:
- Proton
- Neutron
- Bands(Energy)
-
Electron:
- Movement with Probability
- Energy Transition: 发光+导电
Energy Bands
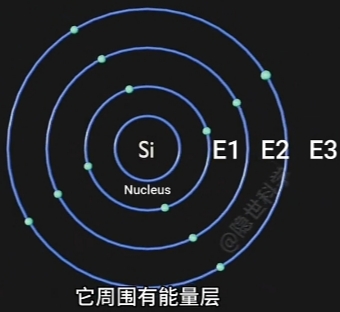
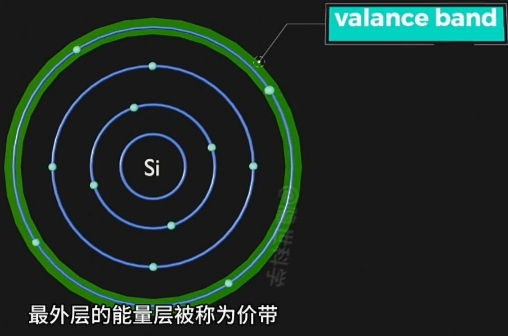
- Nucleus(原子核) 周围存在 Energy Bands(能量层), 称为“价带”;
- Electron(电子) 在 Nucleus(原子核) 周围运动;
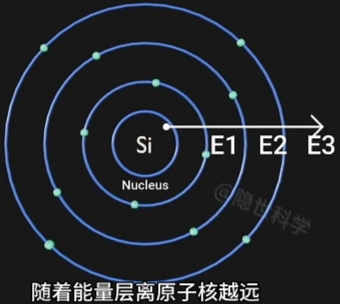
离Nucleus(原子核)越远, Electron(电子) 能量越高. - Electron(电子)的 Position(位置) 的 Measurement(测度) 与 Observation(观测) 有关;
Electron Cloud Hypothesis(电子云假设):
Electron(电子)的 Position(位置)是有Probability的Measure存在;
- Electron(电子) 在 Nucleus(原子核) 周围运动;
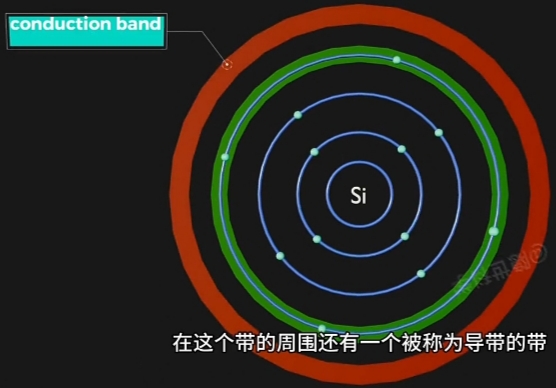
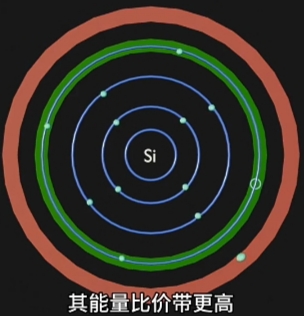
- Energy Bands(能量层) 离 Nucleus(原子核) 越远(半径越大), 能量越高;
- Valance Band(价带), 是 Electron Movement(电子运动)统计上概率稳定的最外层Energy Band;
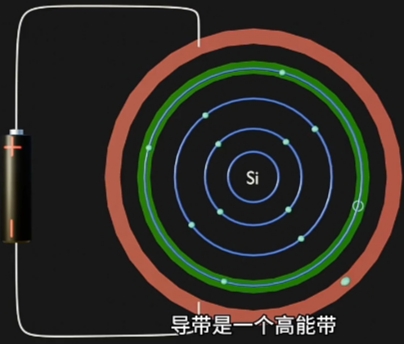
此带上运动的 Electron 是"不自由的", 受Nucleus(原子核)的场力吸引; - Conduction Band(导带), 是 Electron Movement(电子运动)自由(摆脱Nucleus束缚)的Energy Region;
Conduction Band上运动的 Electron 是"自由的",可自由的移动到另一个原子;
- Valance Band(价带), 是 Electron Movement(电子运动)统计上概率稳定的最外层Energy Band;
| 1 | 2 | 3 |
|---|---|---|
 |
 |
 |
| 4 | 5 | 6 |
|---|---|---|
 |
 |
 |
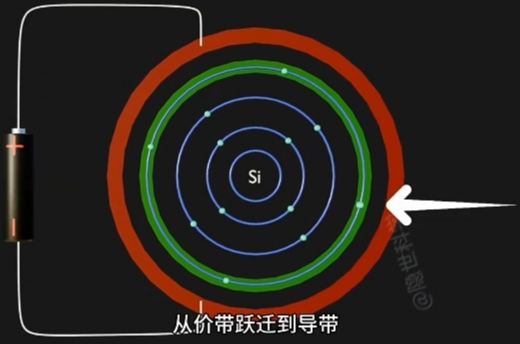
Conduction(导电,施加电场,电子吸收能量)
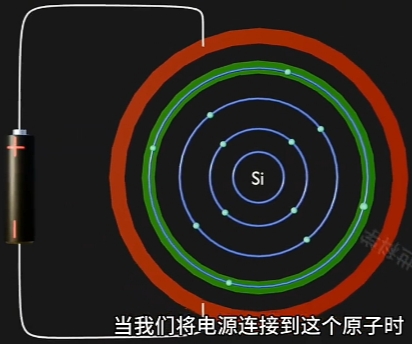
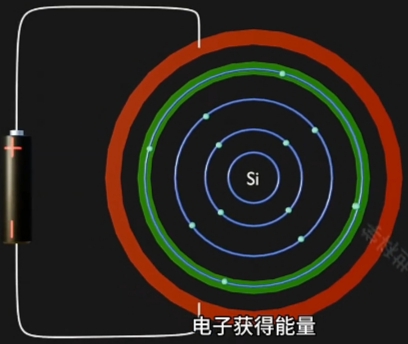
当给原子施加一个电场,Voltage 足够高时:
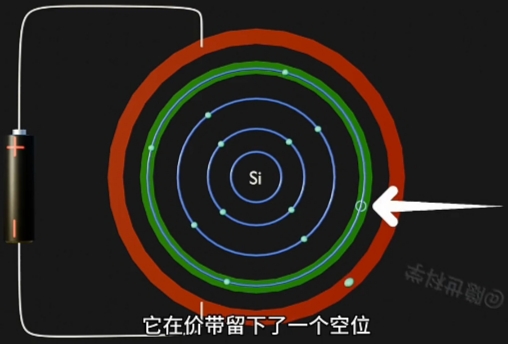
- Conduction Band(导带) 上 的 Electron(电子) 跃迁到Conduction Band,成为自由电子;
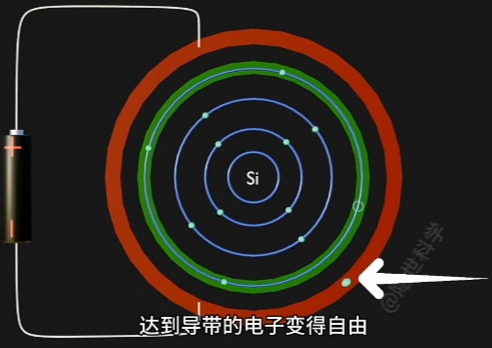
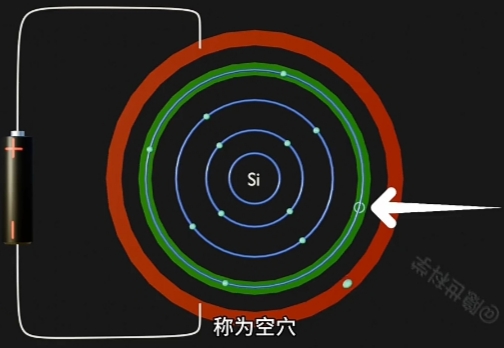
- Conduction Band(导带) 将因为“每个自由电子的跃迁”而形成一个与其对应的“Hole(空穴)”
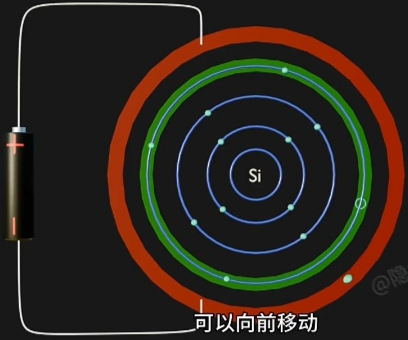
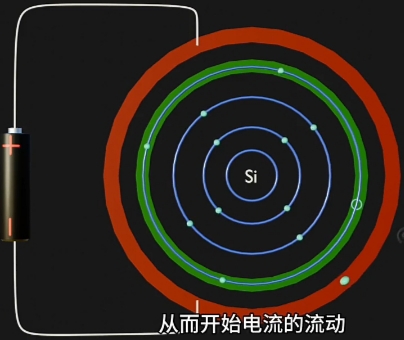
因为 Electron 摆脱 Nucleus, 而在原Atom的Conduction Band上形成对应一个“Hole(空穴)”. - 自由电子的定向移动形成电流, 即电路导通。
电子跃迁 Energy Bands + 自由电子
| 7 | 8 | 9 |
|---|---|---|
 |
 |
 |
自由电子 + 导通电流
| 10 | 11 | 12 | 13 |
|---|---|---|---|
 |
 |
 |
 |
Free Electron(自由电子) + Hole(空穴)形成
| 10 | 11 | 12 | 13 |
|---|---|---|---|
 |
 |
 |
 |
Illumination(电致发光)
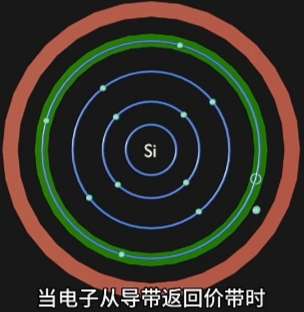
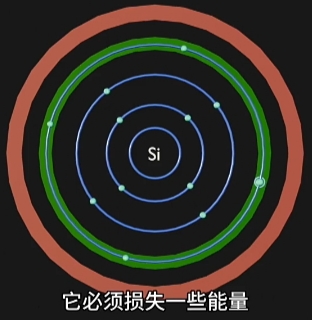
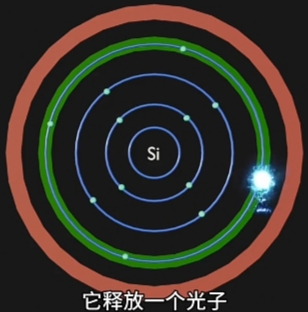
当选择合适“材料”和精密化工、物理、表面工程等工艺,并且施加足够的“电场激发”,
这种合适材料的原子周围的电子将由“高能带”跃迁到“低能量带”,并释放能量;
每种材料的电子释放能量的方式不同:
- 有的释放“光子”,就是“电致发光”,即LED灯发光原理;
- 有的释放?
| 1 | 2 | 3 | 4 |
|---|---|---|---|
 |
 |
 |
 |
深层的科学原理参考 homo-lumo:
https://www.ossila.com/pages/homo-lumo
OLED 就可以使用 以上的“电致发光”原理:
-
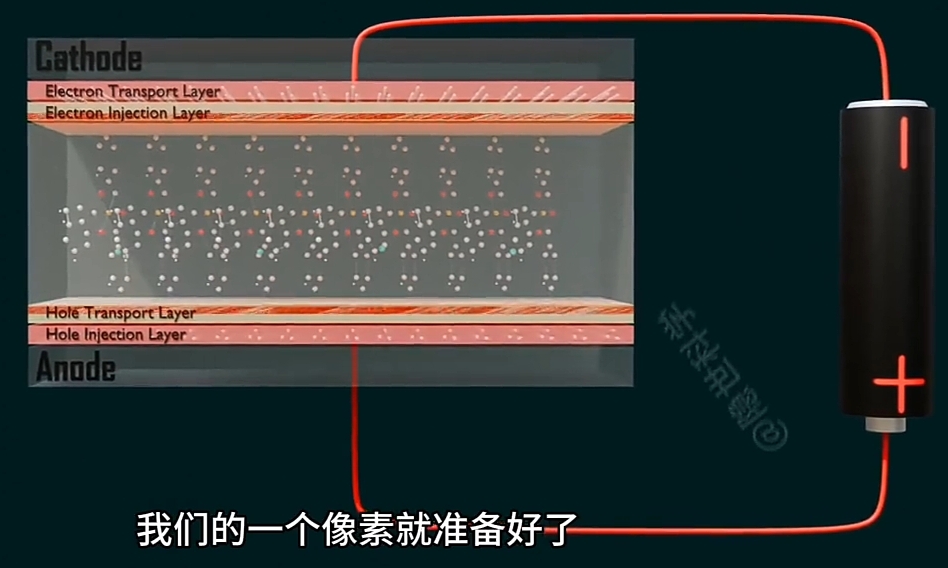
OLED 是由一个个矩阵化的像素(Pixel)构成,
-
每个像素(Pixel)都有一个Emissive Layer(发光层);
-
Emissive Layer(发光层)的材料是“有机化合物”,有特别设计的“分子结构”,
这种特殊的发光分子结构有两个“Energy State(能量级)”, 在有机电化学上:- High Energy State:“Homo(最高占据 分子轨道)”,
HOMO(Highest Occupied Molecular Orbital)
类比“Atom-Electron Model”的 "Valance Band"(价带) - Low Energy State :“Lumo(最低未占据 分子轨道)”
LUMO (Lowest Unoccupied Molecular Orbital)
类比“Atom-Electron Model”的 "Conduction Band"(导带)
- High Energy State:“Homo(最高占据 分子轨道)”,
-
通过精密化工和物理工艺,可以涂覆Emissive Layer,
并将其有机化合物的分子结构的“Anode(阳极)”和 “Cathode(阴极)”以电极引出.
Comparison to the Valence Band (VB) and Conduction Band (CB) in Semiconductors
While HOMO and LUMO are concepts used in molecular systems,
they have analogous counterparts in the context of solid-state semiconductors.
- VB(Valence band) consist of electrons bound tightly enough to atoms,
that they don't move (much) from atom to atom. - CB(Conduction band) consist of electrons that are not bound as strongly,
and can move about, thus allow electricity to be conducted.
They are similar in function to the HOMO and LUMO in organic semiconductors.
Comparison of the HOMO-LUMO levels in Molecular Organic Semiconductors with the Conduction Band-Valence Band in Solid State Semiconductors.
HOMO vs. Valence Band
- The VB edge (VB maximum) is analogous to the HOMO.
- VB maximum is the highest energy level within the valence band that is occupied by electrons at absolute zero temperature.
- VB represents the highest energy band that is fully occupied by electrons in a semiconductor.
Electrons in the VB can be excited to the conduction band, similar to electrons in the HOMO being excited to the LUMO.
LUMO vs. Conduction Band
- The CB edge (CB minimum) is analogous to the LUMO.
- CB minimum refers to the lowest energy level within the conduction band of a material.
- And CB is the lowest energy band that is typically unoccupied in a semiconductor at absolute zero temperature.
- Electrons can be promoted from the VB to the CB, allowing for electrical conduction.
Illustration of HOMO-LUMO Energy Levels
HOMO
The HOMO is the highest energy orbital that is fully occupied by electrons in a molecule.
It represents the highest energy molecular orbital from which an electron can be excited to a higher energy level, such as the LUMO.
The energy of the HOMO is critical in determining the molecule's ability to donate electrons (i.e., its oxidation potential).
LUMO
The LUMO is the lowest energy orbital that is not occupied by electrons.
It represents the frontier molecular orbital that can accept an electron from the HOMO or another electron donor.
The energy of the LUMO is important in determining the molecule's ability to accept electrons (i.e., its reduction potential).
Understanding HOMO and LUMO
HOMO: Highest Occupied Molecular Orbital)
LUMO: Lowest Unoccupied Molecular Orbital)
HOMO and LUMO are fundamental concepts in molecular orbital theory,
also are known as the "frontier molecular orbitals" because they are the highest occupied and lowest unoccupied molecular orbitals, respectively. These orbitals play a crucial role in determining the optical and electronic properties of molecules, such as their ability to absorb light, undergo chemical reactions, and conduct electricity. This information is essential for materials like the non-fullerene acceptor ITIC when determining their suitability for use in solar cells for harnessing the sun's energy.
On This Page
- Molecular Orbital Theory
- Movement of Electrons in HOMO and LUMO Energy Levels
- Determining HOMO and LUMO Energy Levels
- Comparison to the Valence Band (VB) and Conduction Band (CB) in Semiconductors
- Relevance in Organic Electronic Materials and Devices
- Outlook
Molecular Orbital Theory
Orbitals are the space where the electrons are most likely to be found.
Electrons in atoms inhabit atomic orbitals.
The number of electrons in each atom determines the number of electrons within the whole molecule.
Within a molecule the atomic orbitals combine to make up molecule orbitals.
Molecular orbital diagrams show how each atom donates electrons from its atomic orbitals to form molecular orbitals. A molecular orbital diagram showing how atomic orbitals donate electrons to molecular orbitals.
This process of forming molecular orbitals occurs through the addition of atomic wavefunctions.
Wavefunction is a quantum mechanical term that describes the probability of finding an electron.
Wavefunctions can form bonding or antibonding orbitals respectively.
When electrons inhabit the bonding orbital (HOMO), the energy of the molecule decreases, as the bonding orbital is lower in energy than the individual atomic orbitals, and is therefore favourable.
A diagram showing how atomic wavefunctions add or subtract to form bonding and antibonding orbitals.
In molecular orbital theory, these electrons are not restricted to lone pairs or the specific bonds between the atoms. The electrons are delocalized throughout the whole molecule. The molecular orbitals encompass the entire molecule rather than the individual bonds. This provides a more accurate representation of the electronic structure of the molecule. As a result we can get a better understanding of their electronic and chemical properties.
A diagram of the bonds and orbitals that are formed in C2H4
Movement of Electrons in HOMO and LUMO Energy Levels
An electron from the HOMO of a molecule can be promoted to the LUMO when exposed to light or heat.
This forms a new HOMO and LUMO. The movement of electrons within molecules alters its chemical properties.
The promotion of an electron results in the molecule being in its excited state.
In the excited state, the molecule can undergo reactions that it otherwise would not be able to.
Determining HOMO and LUMO Energy Levels
Experimental Methods
PES(Photoelectron Spectroscopy)
The energies of ionized states can be directly determined using photoelectron spectroscopy techniques.
UPS(UV photoelectron spectroscopy) is used to examine occupied levels,including the HOMO,
IPES(Inverse Photoelectron Spectroscopy)
IPES is employed to study unoccupied levels, such as the LUMO.
UV-Vis Spectroscopy
The energy difference between the HOMO and LUMO,
can be estimated from the absorption spectrum of a molecule.
Electrochemical Methods
Cyclic voltammetry is generally preferred to be used to determine the oxidation and reduction potentials, which relate to the HOMO and LUMO energies.
For example, spiro-OMeTAD which is a hole transport layer in perovskite solar cells show:
HOMO at -5.0 eV (calculated by cyclic voltammetry),
LUMO = -2.05 eV (HOMO - Eg).
Computational Methods
DFT(Density Functional Theory)
DFT is a quantum mechanical method that is commonly used to calculate the electronic structure of molecules.
It provides an approximation of HOMO and LUMO energies.
Molecular Orbital Calculations
These are performed using software packages like Gaussian,
which solve the Schrödinger equation for molecules to give orbital energies.
HOMO-LUMO Energy Level Gap vs. Bandgap
The band gap in semiconductors is the energy difference between the VB maximum and CB minimum, determining the electronic and optical properties of the material.
The energy difference between the HOMO and LUMO is called the HOMO-LUMO gap and is generally the lowest energy electronic excitation that is possible in a molecule.
The energy of the HOMO-LUMO gap can tell us about what wavelengths the compound can absorb. Or alternatively, measuring the wavelengths a compound absorbs via experiments like UV-Vis spectroscopy can be used as a measure of the HOMO-LUMO gap.
The HOMO-LUMO gap in molecules plays a similar role but is specific to the discrete energy levels of molecular orbitals rather than continuous energy bands.
Relevance in Organic Electronic Materials and Devices
In the field of organic electronics, HOMO and LUMO energies are critical for understanding and designing materials for devices like organic photovoltaics (OPVs), organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), perovskite photovoltaics, and perovskite LEDs. Their relevance includes:
Charge Transport
The energy levels of the HOMO and LUMO dictate the ease with which charge carriers (electrons and holes) can be injected into the material and transported across it.
An optimal HOMO-LUMO gap along with appropriate energy level alignment typically allows for better charge transport properties, which is crucial for efficient device performance.
Light Absorption and Emission
- In OPVs, the energy gap between the HOMO and LUMO determines the range of wavelengths the material can absorb, which affects the efficiency of light harvesting.
- In OLEDs, the recombination of electrons and holes within the material results in light emission, and the energy gap influences the color of the emitted light.
It is possible to tune the bandgaps in organic materials by compositional engineering such that they have the optimum energy level gap and absorb incoming light efficiently.
Proper alignment minimizes energy barriers, enhances charge extraction and injection, and ultimately improves the overall efficiency and performance of the devices.
Appropriate energy level alignment can be achieved by various methods like compositional tuning, doping, tuning the work-function of the electrodes (in the case of active layer-electrode or charge transport layer-electrode junction), interface engineering by adding an interlayer which improves charge transport, and surface treatments by self assembled monolayers.
Outlook
HOMO and LUMO are fundamental concepts for understanding the optoelectronic properties of organic molecules, especially in the context of organic electronic materials and devices. Their energies can be determined through various experimental and computational methods and are crucial in designing efficient materials for applications such as solar cells, LEDs, and transistors. Understanding the relationship between HOMO-LUMO and CB-VB helps bridge molecular and solid-state physics, providing a comprehensive framework for material design.









